According to the World Health Organization (WHO), AMR threatens the effective prevention and treatment of a wide range of infections and undermines decades of medical progress. Inappropriate antibiotic use, inaccurate laboratory reporting, and lack of standardized antimicrobial susceptibility testing (AST) are key drivers of this crisis.
Clinical microbiology laboratories play a central role in combating AMR. Accurate interpretation of AST results, strict quality control, and real-time surveillance are essential to guide clinicians toward effective therapy and to support national AMR monitoring programs.
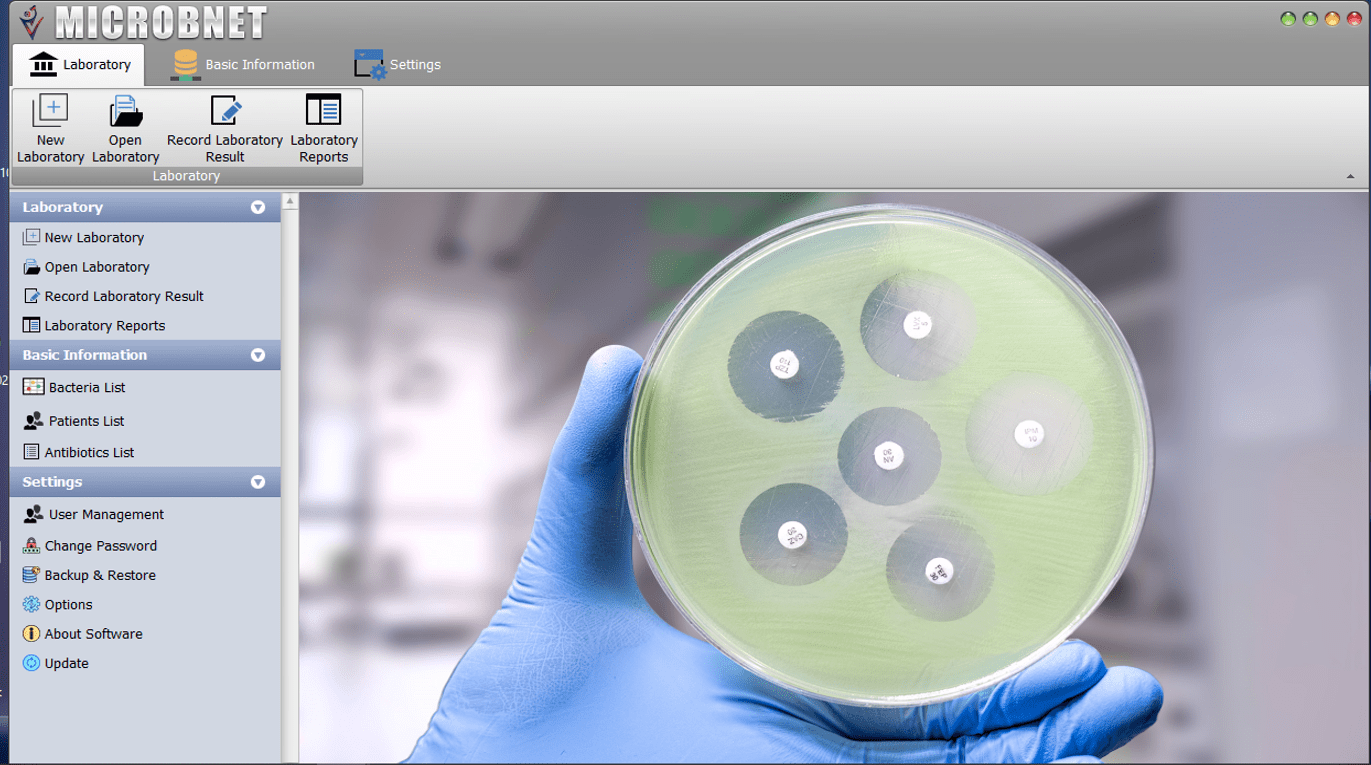
Digital diagnostic intelligence platforms, such as MICROBNET™, address these challenges by standardizing CLSI-based interpretation, preventing reporting errors, automating quality control, and enabling real-time AMR analytics. Strengthening laboratory decision-making is a critical step toward preserving antibiotic effectiveness and protecting public health.